联系人:苏先生
手 机:18612706349
电 话:010-57297418
传 真:010-57297418
地 址:北京市北京经济技术开
发区荣华中路8号院13
号楼10层1111
邮 编:100176
JIP-test和主成分分析(PCA)在植物光合作用研究中的应用
1.快速叶绿素荧光诱导动力学分析(JIP-test)
近二十年来,基于“生物膜能量通量理论”的活体快速叶绿素 a 荧光诱导动力学OJIP曲线和JIP-test分析,由于其无损、精确、快速等特性,已被广泛而成功地用做研究植物生理状态的有力工具(Strasser et
al.,1995, 2004)。植物快速叶绿素荧光诱导曲线(OJIP曲线)中包含着大量关于PSⅡ反应中心原初光化学反应的信息,植物在不同胁迫处理后OJIP曲线会发生特异性变化(Strasser et al., 2004)。OJIP曲线对不同的环境变化极为敏感,例如光胁迫、化学物质影响、热胁迫、低温或冻害、干旱胁迫、重金属或盐胁迫、营养不良、大气二氧化碳或臭氧升高以及病虫害侵染等等。通过对曲线荧光参数的分析,可以知道在环境因子影响下植物光合机构的变化。
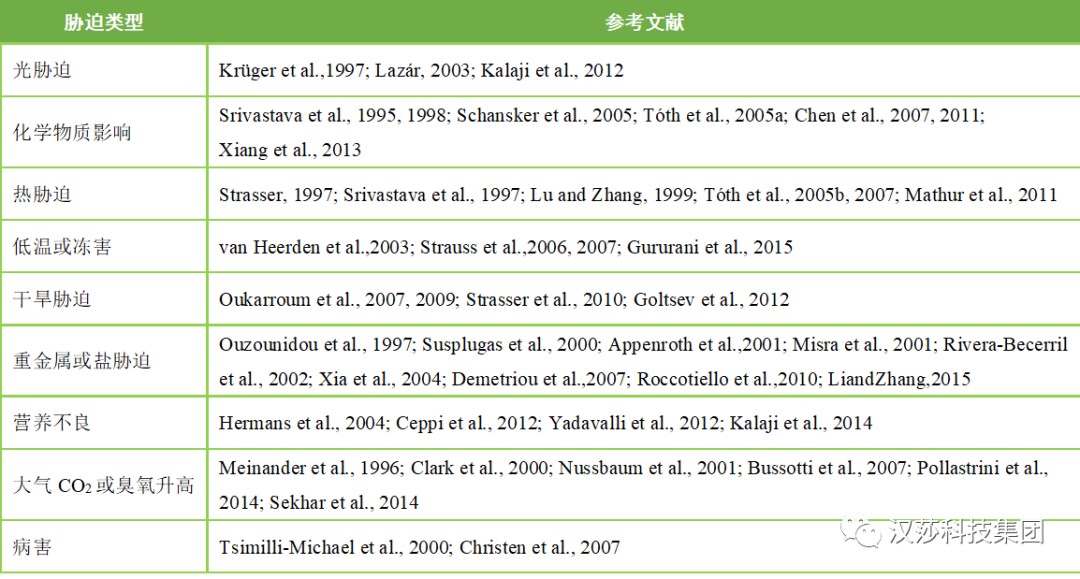
表1.JIP-test在各种植物胁迫研究中的举例
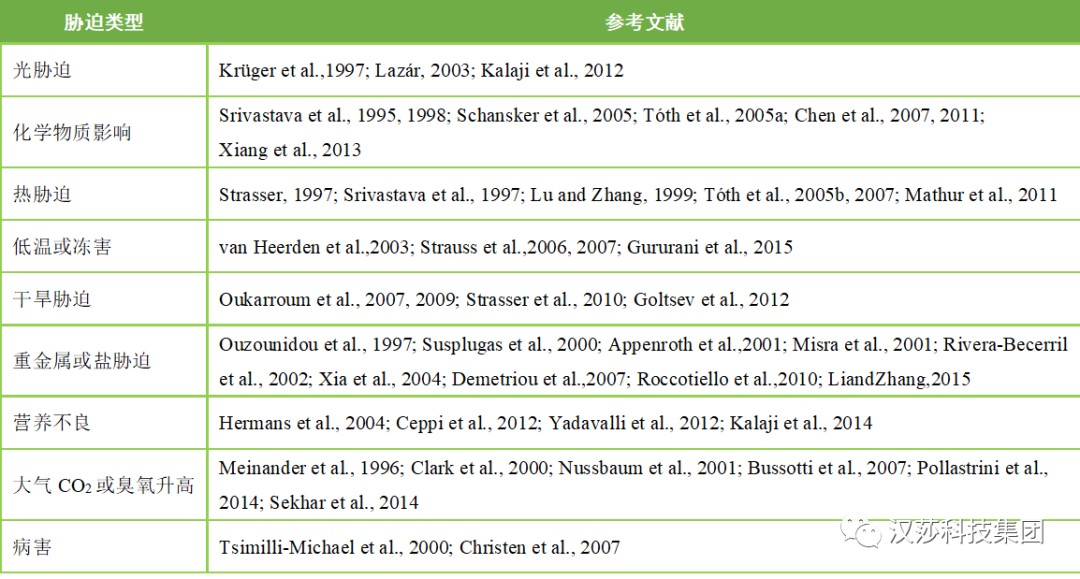
不同环境胁迫JIP-test应用文献目录请移步至公众号底部“技术支持”
→ “文献目录” → “植物效率”
从动力学曲线上可以得到大量的原始数据,为了能更好地反映动力学曲线和被测样品的关系,Strasser
RJ(1995)以生物膜能量流动理论为基础,通过计算能量流和能量比率来衡量在给定物理状态下样品材料内部变化,建立了高度简化的能量流动模型图。
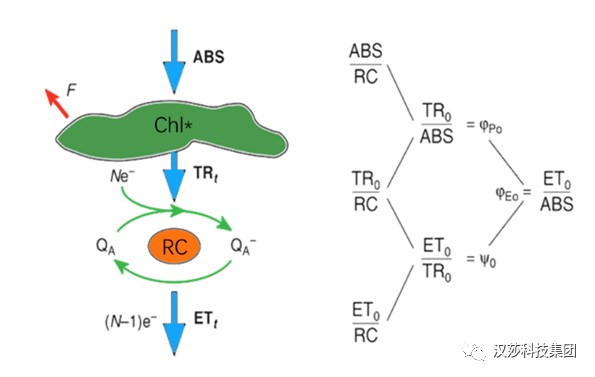
图1. 高度简化的能量在光合器官中的流动模型图(Strasser BJ, Strasser RJ, 1995)
依照能量流动模型,天线色素(Chl)吸收的能量(Absorption, ABS)中的一部分以热能和荧光(F)的形式耗散掉,另一部分则被反应中心(Reaction Centre, RC,在JIP-test中RC指有活性的反应中心)所捕获(Trapping, TR)。在反应中心,激发能被转化为还原能,将QA还原为QA-,后者又可以被重新氧化,从而产生电子传递(electron transport,ET),把传递的电子用于固定二氧化碳或其它途径。
在此基础上发展起来的数据处理称为“JIP-test”(Strasser etal. 1995; Krüger et al. 1997; Strasser et al. 2000, 2004)。JIP-test为我们提供了被测样品的大量信息,如光合器官在不同环境条件下的结构和功能的变化(Strivastava & Strasser1996; Jiang et al. 2003; Hermans et al. 2003; van Heerden et al. 2003, 2004)。
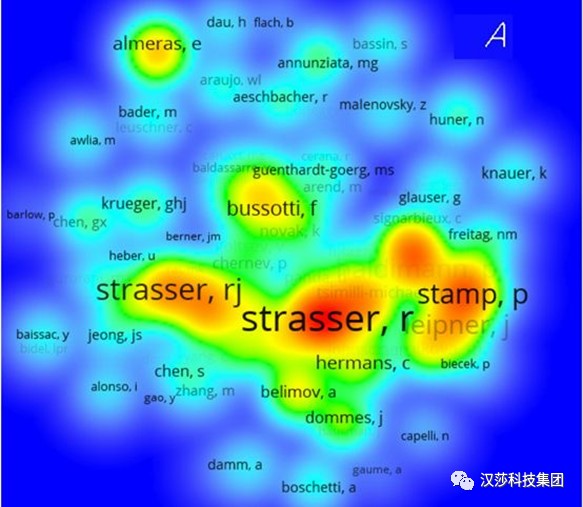
图2. 叶绿素荧光相关联合作者网络(注意R.Strasser和R.J.Strasser是同一个人)。从黄色到红色,协作性更强,中心性更高(K. HU et al, 2020)学术界对JIP-test方法的研究和应用热度在不断增加,而对脉冲调制式(PAM)方法的兴趣在逐渐减弱。这是什么意思?乍一看,一个可能的解释是源于对OJIP动力学实验测量可用性的增加,主要是因为:1)研究者有新的荧光检测方法可用,2)JIP-test已明显证明是基于半经验合理假设的稳健分析工具(robust analysis tool based on semi-empiricalreasonable assumptions)。

图3:Strasser教授和Hansatech初代PEA植物效率分析仪(Rodriguez, 2000年)
由Reto J.Strasser教授发明授权英国Hansatech公司生产的PEA植物效率分析仪系列产品(Handy PEA、M-PEA...)是目前世界上可以完美真实测定OJIP曲线的成熟商品化设备。近20年来,JIP-test方法的不断发展及其在野外应用和实验室研究中的应用呈现出显著的增长趋势。近期发表文章《能量流理论庆祝40年:走向系统生物学概念?》(The energy flux theory celebrates 40 years: toward a systems biology concept?" Photosynthetica, April 2019, 57(2):521-522.)详细阐述了这一研究热点趋势。2019年末,国际光合作用研究杂志(Photosynthetica)推出荣耀特刊,刊发30余篇荣耀文章以表彰纪念Strasser教授在JIP-test理论方向做出的卓越贡献。
荣耀特刊文献预览及下载请点击以下链接文章:
2.主成分分析(PCA)简介
主成分分析(Principal Components Analysis)也称主分量分析,旨在利用“降维”的思想,把多指标转化为少数几个综合指标。在许多研究领域中,通常需要对含有多个变量的数据进行观测,收集大量数据后进行分析寻找规律。多变量大数据集为研究提供了丰富的信息,而在多数情况下,许多变量之间可能存在相关性,从而增加了问题分析的复杂性。如果分别对每个指标进行分析,分析往往是孤立的,不能完全利用数据中的信息,因此盲目减少指标会损失很多有用的信息,从而产生错误的结论。鉴于各变量之间存在一定的相关关系,因此可以考虑将关系紧密的变量变成尽可能少的新变量,使这些新变量是两两不相关的,那么就可以用较少的综合指标分别代表存在于各个变量中的各类信息。主成分分析PCA就属于这类降维算法,将高维度的数据保留下最重要的一些特征,去除噪声和不重要的特征,从而实现提升数据处理速度的目的。
图4a. 数据点降维的信息损失与矫正:X轴投影
如何降维?我们以最简单的二维转一维为例,如图4中就是把二维平面上不同位置上的点投影到同一条直线上(X轴或Y轴)。但是仔细观察前两个图,我们就会发现,有些点在投影过后,位置是重合的,也就是说,存在不同的点在压缩过后表示的信息是完全一样的。投影到x轴,有两个点重合;投影到y轴,有三个点重合。
图4b. 数据点降维的信息损失与矫正:Y轴投影
这就是当所有点集中至一条轴上时,另一维度或另一轴上的信息就会丢失,这是不可逆的过程,这一信息的损失也是必然的。这不是我们想要的结果,最终我们还是希望点与点之间间隔尽可能的远,保留的信息尽可能的多,让所有的点能够尽可能的进行区分。
图4c. 数据点降维的信息损失与矫正:X/Y轴矫正
最好的结果应该是我们依然选择了某个直线,并把点投影到这条直线上,但是点之间没有重合,点与点的间隔也比较远。看到这里,我们就知道PCA到底要做什么了。没错!就是找到这条直线,并求出投影到这条直线的点的坐标(当然二维降一维是直线,三维降二维就是平面了,更多维度也是类似的)。
3.主成分分析在JIP-test中的应用
主成分分析(PCA)是深度分析JIP-test众多荧光参数的有效方法。通过PCA对JIP-test荧光参数进行二次处理,对其数量、精度和复杂性进行分析,可以识别荧光参数大数据中内的隐藏信息,而传统方法则是无法有效进行的(Samborska et
al.2014)。
使用PEA系列植物效率分析仪,每个样品仅需2秒钟,即可获得完整OJIP曲线和50多个荧光参数,包括(i)OJIP曲线特征位点FJ、FI、Area等,(ii)比活性参数ABS/RC、TRM/RC等,(iii)性能指数PIABS、PItotal等和(iiiii)推动力DFABS等。JIP-test每个荧光参数并不是完全独立的,因为JIP-test荧光参数是根据荧光瞬态曲线点计算的,其中一些参数由于其数学表达式(如φDo和φPo)而具有很高的相关性。通过主成分分析PCA评估植物在不同环境下的生理或胁迫效应,以确定对植物光合生理反应最敏感的参数,这种方法允许将一组测量参数转换成较少的变量,以确定植物生理状态的变化(Jolliffe,2002;
Legendre and Legendre 2012; Goltsev etal. 2012)。
如图5中JIP-test荧光数据来自于不同生长年龄短柄草(随着生长年龄的增大,其林分密度随之增大)。首先第一PCA轴(Dim1)向上,两个极值分别为:VI和单位PSⅡ活性反应中心比通量参数(TRo/RC、ETo/RC、REo/RC)。
同时第二PCA轴(Dim2)向上,可以看到参数Fv/Fo和PSⅡ原初最大量子产率(ΦPo)的增大。
通过这种方法,我们发现了四个最重要的参数(而不是最初的54个)来描述光合机构的状态,它们与短柄草的林分密度的增加显著相关。
图6中对不同施肥处理的玉米JIP-test荧光数据进行PCA分析,使其分为了5个分离簇。第一类为对照组和缺磷植株。此簇位于Comp1和Comp2均为正值的第一象限,结果表明与对照组相比,缺磷处理对玉米光合机构的影响不显著。第二类是均匀分布在坐标系原点附近的缺氮、缺镁和缺硫样品。缺氮、缺硫植株的参数点略有向正方向移动,缺镁植株的参数点向负方向移动。这意味着尽管JIP-test荧光参数变化具有相似性,但仍有足够的特征可用作区分组内样本的荧光表型标记。第三类主要由植物缺钾样品组成,位于Comp1和Comp2的负区。这意味着玉米中钾的缺乏可以通过JIP-test来很容易地确定。第四和第五个簇是由缺铁和缺钙植株形成的,即当玉米缺铁或缺钙时,具有相似的JIP-test参数,并且它们与其他缺肥处理有很好的分离。
图7. 不同环境条件下5个玉米杂交种叶片JIP试验参数变异性的主成分分析:对照(C)、弱光(LL)、田间(F)、冷(Co)、热(H)和高温(SH)(Frani M et al. 2020)
图7为不同环境条件下5个玉米杂交种叶片JIP-test参数变异性的主成分分析:前三主成分占总方差的95.9%,选择的14个参数对环境效应的敏感性不同,因而对主成分形成的贡献也不同(数据见原文)。
所有五种处理都是独立的簇,并位于坐标系的不同区域。SH处理对玉米植株的热胁迫最为分散,通过JIP-test荧光参数的变化可以看出热胁迫对玉米植株的严重性。
PC1与DIo/RC(0.98)和RC/ABS(–0.96)的相关性最强,因此可以认为PC1是一个功能反应中心的量度,其两端极值处理组为C和SH。与PC2两极相关性最强的参数为(VJ,-0.90)和ΨEo(0.87)。
在第二主成分两端的是F、Co和LL处理组,其中LL和Co的主要特征参数是VJ和VI,F处理组的特征是解释电子传递通量的ΨEo和ETo/RC。在最近对几种植物的环境影响分类的研究中,也显示了相似的JIP-test参数分组(Bussotti et al. 2020)。
此例中PIABS似乎只提供了一个轴向的分类,而其他JIP-test荧光参数可用于检测各个环境条件下对玉米的特定影响。例如,第一主成分的相对侧显示了玉米植株受到的两个环境极值:冷胁迫处理组(Co)-主要由VJ和VI参数表征,而高温胁迫处理组(SH)-主要由K、Mo、REo/RC和DIo/RC表征。
Stirbet(Stirbet et al. 2018)等人也证实了这一点,同时建议设计新参数以表征已知特定条件反应的JIP-test参数。同时Galic等人(Galic et al. 2019)表明,PIABS可以有效地用于热胁迫环境下的粮食产量选择。
总的来说通过PCA我们可以分类植物对各种环境因素的不同反应:(i)找到特定处理下植物样品OJIP曲线发生的特异性变化(ii)筛选出发生显著变化的JIP-test荧光参数及其变化特征,可更好对植物样品光合机构发生的变化(伤害)进行定位分析,如PSⅡ供体侧/受体测或PSⅡ活性中心等。(iii)我们还可以将JIP-test荧光数据与其他环境数据或生理参数进行聚类结合(Goltsev et al. 2012)。(iv)此外Tyystjärvi等人应用PCA等人工智能方法分析不同类型光照(低光强、饱和脉冲、远红色等)激发的JIP-test荧光数据,可识别植物物种(Tyystjärvi et al. 1999; Keränen et al. 2003; Codrea et al. 2003;Kirova et al. 2009)。(v)Kalaji等人利用JIP-test、主成分分析(PCA)和一种新的机器学习方法建立了一种无创检测和监测大田条件下油菜籽微量和大量营养素缺乏的方法(Kalaji et al. 2017)。鉴于篇幅限制,我们将在下期文章中筛选数篇应用PCA方法分析JIP-test荧光数据具有代表性的文章进行详细介绍,期待您的关注,谢谢!
4.引用文献
[1] Appenroth, K.J., Stöckel, J., Srivastava, A.,Strasser, R.J., 2001. Multiple effects of chromate on the photosyntheticapparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescencemeasurements. Environ. Pollut. 115, 49–64.
[2] Bussotti F, Gerosa G, Digrado A, Pollastrini M, 2020.Selection of chlorophyll fluorescence parameters as indicators of photosyntheticefficiency in large scale plant ecological studies. Ecol Indic 108: 105686.
[3] Bussotti, F., Strasser, R.J., Schaub, M., 2007.Photosynthetic behavior of woody species under high ozone exposure probed withthe JIP-test: a review. Environ. Pollut. 147, 430–437.
[4] Ceppi, M.G., Oukarroum, A., Cicek, N., Strasser,R.J., Schansker, G., 2012. The IP amplitude of the fluorescence rise OJIP issensitive to changes in the photosystem I content of leaves: a study on plantsexposed to magnesium and sulfate deficiencies, drought stress and salt stress. Physiol.Plant 144, 277–288.
[5] Chen, S.G., Xu, X.M., Dai, X.B., Yang, C.L., Qiang,S., 2007. Identification of tenuazonic acid as a novel type of naturalphotosystem II inhibitor binding in QB-site of Chlamydomonasreinhardtii. Biochim. Biophys. Acta 1767, 306–318.
[6] Chen, S.G., Zhou, F.Y., Yin, C.Y., Strasser, R.J.,Qiang, S., Yang, C.L., 2011. Application of fast chlorophyll a fluorescencekinetics to probe action target of 3-acetyl-5-isopropyltetramic acid. Environ.Exp. Bot. 71, 269–279.
[7] Christen, D., Schönmann, S., Jermini, M., Strasser,R.J., Défago, G., 2007. Characterization and early detection of grapevine (Vitisvinifera) stress responses to esca disease by in situ chlorophyllfluorescence and comparison with drought stress. Environ. Exp. Bot. 60,504–514.
[8] Clark, A.J., Landolt, W., Bucher, J.B., Strasser,R.J., 2000. Beech (Fagus sylvatica) response to ozone exposure assessedwith a chlorophyll a fluorescence performance index. Environ. Pollut.109, 501–507.
[9] Codrea C, Aittokallio T, Keränen M et al(2003) Feature learning with a genetic algorithm for fluorescencefingerprinting of plant species. Pattern Recognit Lett 24:2663–2673.
[10] Demetriou, G., Neonaki, C., Navakoudis,
E.,Kotzabasis, K., 2007. Salt stress impact on the molecular structure
andfunction of the photosynthetic apparatus—the protective role
of polyamines. Biochim.Biophys. Acta 1767, 272–280.
[11] Frani M, Jambrovi A, Zduni Z, et al. Photosyntheticproperties of maize hybrids under different environmental conditions probed bythe chlorophyll a fluorescence[J]. Maydica, 2020, 64(3):M25.
[12] Galić V, Mazur M, Šimić D, Zdunić Z, Franić M, 2019.Plant biomass in salt-stressed young maize plants can be modelled with photosyntheticperformance. Photosynthetica 57: 9-19.
[13] Goltsev V, Zaharieva I, Chernev P et al (2012)Drought-induced modifications of photosynthetic electron transport in intactleaves: analysis and use of neural networks as a tool for a rapid non-invasiveestimation. Biochim Biophys Acta-Bioenerg 1817:1490–1498.
[14] Gururani, M.A., Venkatesh, J., Ganesan, M.,Strasser, R.J., Han, Y., Kim, J.I., Lee, H.Y., Song, P.S., 2015. In vivoassessment of cold tolerance through chlorophyll-a fluorescence in transgeniczoysiagrass expressing mutant phytochrome A. PLoS One 10, e0127200.
[15] Hermans C, Smeyers M, Rodriguez RM, Eyletters M,Strasser RJ, Delhaye JP (2003). Quality assessment of urban trees: Acomparative study of physiological characterization, airborne imaging and onsite of fluorescence monitoring by the OJIP-test. J Plant Physiol, 160:81–90.
[16] Hermans, C., Johnson, G.N., Strasser, R.J.,Verbruggen, N., 2004. Physiological characterisation of magnesium deficiency insugar beet: acclimation to low magnesium differentially affects photosystems Iand II. Planta 220, 344–355.
[17] Hu, K., Govindjee, G., Tan, J., Xia, Q., Dai, Z. andGuo, Y. Co-author and co-cited reference network analysis for chlorophyllfluorescence research from 1991 to 2018. Photosynthetica, 2020, vol. 58,iss. 1, p. 110-124.
[18] Jiang CD, Gao HY, Zou Q (2003). Changes of donorand accepter side in photosystem II complex induced by iron deficiency inattached soybean and maize leaves. Photosynthetica, 41: 267–271.
[19] Jolliffe, I.T., 2002. Graphical representation ofdata using principal components. In: Jolliffe, I.T. (Ed.), Principal ComponentAnalysis, Springer Series in Statistics. Springer, New York, pp. 78-110.
[20] Kalaji H M, BaBa W , Gediga K , et al. Chlorophyllfluorescence as a tool for nutrient status identification in rapeseedplants[J]. Photosynthesis Research, 2017.
[21] Kalaji H M, Oukarroum A, Alexandrov V, et al.Identification of nutrient deficiency in maize and tomato plants by in vivochlorophyll a fluorescence measurements[J]. Plant Physiology &Biochemistry, 2014, 81:16-25.
[22] Kalaji, H.M., Carpentier, R., Allakhverdiev, S.L.,Bosa, K., 2012. Fluorescence parameters as early indicators of light stress inbarley. J. Photochem. Photobiol. B: Biol. 112, 1–6.
[23] Keränen M, Aro EM, Tyystjärvi E, Nevalainen O(2003) Automatic plant identification with chlorophyll fluorescencefingerprinting. Precis Agric 4:53–67.
[24] Kirova M, Ceppi G, Chernev P et al (2009)Using artificial neural networks for plant taxonomic determination based onchlorophyll fluorescence induction curves. Biotechnol Biotechnol Equip23:941–945.
[25] Krüger, G.H.J., Tsimilli-Michael, M., Strasser,R.J.,1997. Light stress provokes plastic and elastic modifications instructureand function of photosystem II in camellia leaves. Physiol. Plant. 101,265–277.
[26] Lazár, D., 2003. Chlorophyll a fluorescence riseinduced by high light illumination of dark-adapted plant tissue studied bymeans of a model of photosystem II and considering photosystem IIheterogeneity. J. Theor. Biol. 220, 469–503.
[27] Legendre P, Legendre L (2012) Numerical ecology,3rd edn. Elsevier, Amsterdam.
[28] Li, X., Zhang, L., 2015. Endophytic infectionalleviates Pb2+ stress effects on photosystem II functioning of Oryzasativa leaves. J. Hazard. Mater. 295, 79–85.
[29] Lu, C.M., Zhang, J.H.,1999. Heat-induced multipleeffects
on PSII in wheat Plants. J. Plant Physiol. 156, 259–265.
[30] Mathur, S., Allakhverdiew, S.I., Jajoo,A.,2011.Analysis of high
temperature stress on the dynamic of antenna size andreducing side
heterogeneity of photosystem II in wheat leaves (Triticumaestivum). Biochim.
Biophys. Acta 1807, 22–29.
[31] Meinander, O., Somersalo, S., Holopainen, T.,Strasser, R.J., 1996. Scots pines after exposure to elevated ozone and carbondioxide probed by reflectance spectra and chlorophyll a fluorescencetransients. J. Plant Physiol. 148, 229–236.
[32] Misra, A.N., Srivastava, A., Strasser, R.J., 2001.Utilization of fast chlorophyll a fluorescence technique in assessing thesalt/ion sensitivity of mung bean and Brassica seedlings. J. Plant Physiol.158, 1173–1181.
[33] Nussbaum, S., Geissmann, M., Eggenberg, P.,Strasser, R.J., Fuhrer, J., 2001. Ozone sensitivity in herbaceous species asassessed by direct and modulated chlorophyll fluorescence techniques. J.Plant Physiol. 158, 757–766.
[34] Oukarroum, A., Madidi, S. E., Schansker, G.,Strasser, R.J., 2007. Probing the responses of barley cultivars (Hordeumvulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress andre-watering. Environ. Exp. Bot 60, 438–446.
[35] Oukarroum, A., Schansker, G., Strasser, R.J., 2009.Drought stress effects on photosystem I content and photosystem IIthermotolerance analyzed using Chl a fluorescence kinetics in barley varietiesdiffering in their drought tolerance. Physiol. Plant 137, 188–199.
[36] Ouzounidou, G., Moustakas, M., Strasser, R.J.,1997. Sites of action of copper in the photosynthetic apparatus of maizeleaves: kinetics analysis of chlorophyll fluorescence, oxygen evolution,absorption changes and thermal dissipation as monitored by photoacousticsignals. Aust. J. Plant Physiol. 24, 81–90.
[37] Pollastrini, M., Desotgiu, R., Camin, F., Ziller,L., Gerosa, G., Marzuoli, R., Bussotti, F., 2014. Severe drought eventsincrease the sensitivity to ozone on poplar clones. Environ. Exp. Bot.100, 94–104.
[38] Pontes. D, Ontes, M., Rodriguez, R. and Santiago,E.F. Letter to The Editor. The energy flux theory celebrates 40 years: toward asystems biology concept? Photosynthetica, 2019, vol. 57, iss. 2, p.521-522.
[39] Rivera-Becerril, F., Calantzis, C., Turnau, K.,Caussanel, J., Belimov, A. A., Gianinazzi,S., Strasser, R.J., Gianinazzi-Pearson,V., 2002. Cadmium accumulation and buffering of cadmium-induced stress byarbuscular mycorrhiza in three Pisum sativum L. genotypes. J. Exp.Bot. 53, 1177–1185.
[40] Roccotiello, E., Manfredi, A., Drava, G., Minganti,V., Mariotti, M.G., Berta, G., Cornara, L., 2010. Zinc tolerance andaccumulation in the ferns Polypodium cambricum L. and Pteris vittataL. Ecotoxicol. Environ. Saf. 73, 1264–1271.
[41] Samborska IA, Alexandrov V, Sieczko L et al (2014)Artificial neural networks and their application in biological and agriculturalresearch. Sigpost Open Access J Nano Photo Bio Sciences 2:14–30.
[42] Schansker, G., Tóth, S.Z., Strasser, R.J., 2005.Methylviolegen and dibromothymoquinone treatments of pea leaves reveal the roleof photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta1706, 250–261.
[43] Sekhar, K.M., Rachapudi, V.S., Mudalkar, S., Reddy,A.R., 2014. Persistent stimulation of photosynthesis in short rotation coppicemulberry under elevated CO2 atmosphere. J. Photochem. Photobiol.B: Biol. 137, 21–30.
[44] Srivastava, A., Guissé, B., Greppin, H., Strasser,R.J., 1997. Regulation of antenna structure and transport in photosystem II of Pisumsativum under elevated temperature probed by fast polyphasic chlorophyll afluorescence transient: OKJIP. Biochim. Biophys. Acta 1320, 95–106.
[45] Srivastava, A., Jüttner, F., Strasser, R.J., 1998.Action of the allelochemical, fischerellin A, on photosystem II. Biochim.Biophys. Acta 1364, 326–336.
[46] Srivastava, A., Strasser, R.J., Govindjee, 1995.Differential effects of dimethylbenzoquinone and dichlorobenzoquinone onchlorophyll fluorescence transient in spinach thylakoids. J. Photochem.Photobiol. B: Biol. 31, 163–169.
[47] Stirbet A, Lazár D, Kromdijk J, Govindjee, 2018. Chlorophylla fluorescence induction: Can just a one-second measurement be used to quantifyabiotic stress responses? Photosynthetica 56: 86-104.
[48] Strasser BJ, Strasser RJ (1995). Measuring fastfluorescence transients to address environmental questions: The JIP test. In:Mathis P (eds). Photosynthesis: from Light to Biosphere. Dordrecht: KAPPress, Vol 5: 977-980.
[49] Strasser RJ, Srivastava A, Tsimilli-Michael M(2000). The fluorescence transient as a tool to characterize and screenphotosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds). ProbingPhotosynthesis: Mechanism, Regulationand Adaptation. London: Taylor andFrancis Press, 445–483.
[50] Strasser RJ, Tsimill-Michael M, Srivastava A(2004). Analysis of the chlorophyll a fluorescence transient. In: PapageorgiouG, Govindjee(eds). Advances in Photosynthesis and Respiration.Netherlands: KAP Press, 1–42.
[51] Strasser, B.J., 1997. Donor side capacity ofPhotosystem II probed by chlorophyll a fluorescence transients. Photosynth.Res. 52, 147–155.
[52] Strasser, R.J., Tsimilli-Michael, M., Qiang, S.,Goltsev, V., 2010. Simultaneous in vivo recording of prompt and delayedfluorescence and 820-nm reflection changes during drying and after rehydrationof the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta1313–1326.
[53] Strauss, A.J., Krüger, G.H.J., Strasser, R.J., vanHeerden, P.D.R., 2006. Ranking of dark chilling tolerance in soybean genotypesprobed by the chlorophyll a fluorescence transient O-J-I-P. Environ. Exp.Bot. 56, 147–157.
[54] Strauss, A.J., Krüger, G.H.J., Strasser, R.J., vanHeerden, P.D.R., 2007. The role of low soil temperature in the inhibition ofgrowth and PSII function during dark chilling in soybean genotypes ofcontrasting tolerance. Physiol. Plant 131, 89–105.
[55] Strivastava A, Strasser RJ (1996). Stress andstress management of land plants during a regular day. J Plant Physiol,148: 445–455.
[56] Susplugas, S., Srivastava, A., Strasser, R.J.,2000. Changes in the photosynthetic activities during several stages ofvegetative growth of Spirodela polyrhiza: effect of chromate. J. PlantPhysiol. 157, 503–512.
[57] Tóth, S.Z., Schansker, G., Garab, G., Strasser,R.J., 2007. Photosynthetic electron transport activity in heat-treated barleyleaves: the role of internal alternative electron donors to photosystem II. Biochim.Biophys. Acta 1767, 295–305.
[58] Tóth, S.Z., Schansker, G., Kissimon, J., Kovacs,L., Garab, G., Strasser, R.J., 2005b. Biophysical studies of photosystemII-related recovery processes after a heat pulse in barley seedlings (Hordeumvulgare L.). J. Plant Physiol. 162, 181–194.
[59] Tóth, S.Z., Schansker, G., Strasser, R.J., 2005a.In intact leaves, the maximum fluorescence level (FM) isindependent of the redox state of the plastoquinone pool: a DCMU-inhibitionstudy. Biochim. Biophys. Acta 1708, 275–282.
[60] Tsimilli-Michael, M., Eggenberg, P., Biro, B.,Köves-Pechy, K., Vörös, I., Strasser, R.J., 2000. Synergistic and antagonisticeffects of arbuscular mycorrhizal fungi and Azospirillum and Rhizobiumnitrogen-fixers on the photosynthetic activity of alfalfa, probed by thepolyphasic chlorophyll a fluorescence transient O-J-I-P. Appl. Soil Ecol.15, 169–182.
[61] Tyystjärvi E, Koski A, Keränen M, Nevalainen O(1999) The Kautsky curve is a built-in barcode. Biophys J 77:1159–1167.
[62] van Heerden PDR, Strasser RJ, Krüger GHJ (2004).Reduction of dark chilling stress in N 2 -fixing soybean by nitrate asindicated by chlorophyll a fluorescence kinetics. Physiol Plant, 121:239–249.
[63] van Heerden PDR, Tsimilli-Michael M, Krüger GHJ,Strasser RJ (2003). Dark chilling effects on soybean genotypes duringvegetative development: parallel studies of CO2 assimilation,chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation. PhysiolPlant, 117: 476–491.
[64] Xia, J.R., Li, Y.J., Zou, D.H., 2004. Effects ofsalinity stress on PSII in Ulva lactuca as probed by chlorophyll fluorescencemeasurements. Aquat. Bot. 80, 129–137.
[65] Xiang, M.M., Chen, S.G., Wang, L.S., Dong, Z.Y.,Huang, J.H., Zhang, Y.X., Strasser, R.J., 2013. Effect of vulculic acidproduced by Nimbya alternantherae on the photosynthetic apparatus of Alternanthera.philoxeroides. Plant Physiol. Biochem 65, 81–88.
[66] Yadavalli, V., Neelam, S., Rao, A.S.V.C., Reddy,A.R., Subramanyam, R., 2012. Differential degradation of photosystem I subunitsunder iron deficiency in rice. J. Plant Physiol. 169, 753–759.



暂无信息
